Audit findings are a major pain point for life sciences companies, and non-compliance could result in significant penalties. Discover proactive strategies for minimizing audit findings to achieve compliance at scale.
Understanding audit findings in life sciences
To remain compliant, any organization within the life sciences sector must undertake audits. This process involves investigating, verifying, and reviewing activities related to producing and distributing products. Auditors look at everything from suppliers to manufacturing processes to documentation.
Audit findings arising from this are discrepancies against regulations or other standards. Following an audit, addressing these findings is important to ensure ongoing compliance.
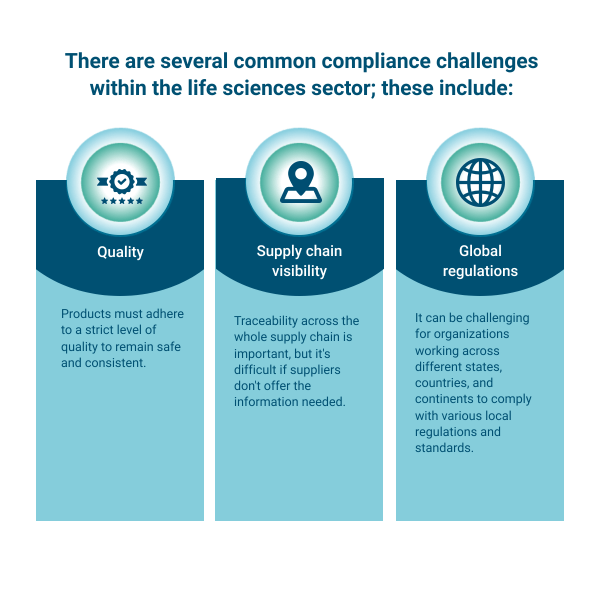
There are several common compliance challenges within the life sciences sector; these include:
- Quality: Products must adhere to a strict level of quality to remain safe and consistent.
- Supply chain visibility: Traceability across the whole supply chain is important, but it’s difficult if suppliers don’t offer the information needed.
- Global regulations: It can be challenging for organizations working across different states, countries, and continents to comply with various local regulations and standards.
The importance of proactive compliance
Proactive compliance involves understanding the regulations and laws affecting a business and its products. With a proactive approach, an organization can identify and address issues before they appear as audit findings.
An audit is a necessary part of compliance, but a large list of audit findings can significantly impact companies in the life sciences. For example, there could be financial penalties for non-compliance, but these organizations could also face reputational damage, especially if they consistently face significant issues.
Fundamental strategies for reducing audit findings
A proactive approach, with strong internal controls and regular self-assessments, can help reduce audit findings. This process ensures everyone in the organization is working toward the same goals with compliance and quality in mind.
Risk management
Careful risk assessment is a key part of the life sciences industry. Understanding the factors that can lead to audit findings will help an organization better understand where they might best be at risk. For example, outlining potential threats to safety, efficiency, and quality can help an organization see where they might encounter compliance issues.
A robust risk management framework will ensure an organization is clear on potential compliance risks. This starts by understanding regulatory requirements and how they might impact business operations.
Creating a risk matrix can help an organization understand the severity and frequency of those potential risks. This can then act as a guideline for proactive action to help reduce the most severe, frequent risks. For instance, if the risk matrix makes it clear that a single supplier has a high chance of risk, the organization can take action. This not only reduces risk to the company and its products but can also reduce audit findings.
As part of creating this framework, the organization should implement the appropriate risk management tools. This will ensure the risk framework and proactive action align with regulations, industry standards, and wider business goals.
To learn more about how ETQ Reliance can help improve compliance at scale, look here.
Quality management measures
Quality management practices can prevent compliance issues and reduce audit findings. That’s because many standards are rooted in quality. In focusing on the quality of a product, an organization can improve regulatory compliance and streamline the auditing process. Using a quality management system (QMS), it’s possible to link quality, health, safety directly, and the environment with the regulations and standards affecting those areas.
With the right QMS software, certain processes can be automated to help reduce the risk of audit findings. For example, a large part of quality management is document control, for which industry standards and regulations also have clear criteria. Automating document control processes to improve efficiency and reduce errors helps an organization become more compliant.
Another area where a QMS can help an organization remain compliant is through continuous monitoring. With real-time reporting, risks, and compliance issues can be identified without needing a full internal audit. This proactive approach ensures life sciences companies can achieve compliance at scale.
Leveraging technology for compliance at scale
Achieving compliance at scale within the life sciences industry requires a proactive approach. This method focuses on real-time reporting, continuous improvement, and risk management tools.
ETQ’s role in enhancing compliance
ETQ Reliance® supports risk management through advanced analytics to help foster a culture of continuous improvement. This, in turn, enhances compliance, allowing an organization to track and manage compliance-related tasks in a single place. This promotes compliance proactively while ensuring important tasks don’t slip through the net.
ETQ Reliance is a software program that can be helpful for organizations. In particular, it can help businesses in heavily regulated industries, such as life sciences, stay on top of regulatory changes. ETQ Reliance includes tools to automate compliance processes by identifying, accessing, and evaluating laws, regulations, and organizational requirements. It then ensures all documentation is stored in a central repository to streamline the process of viewing, updating, approving, and archiving anything related to these regulations.
With these digital tools centralizing quality and compliance while automating necessary processes, managing huge amounts of data is possible. This is a great way to manage compliance at scale for life sciences companies that are growing rapidly across different sectors and territories.
Implementing effective compliance programs
A compliance program can reduce the likelihood of audit findings. When risk and audit management processes align with internal goals, everyone in the organization can be on board. This alignment makes compliance a natural part of everything the organization does. Regular audits throughout the year make it possible to see which activities have the most impact on audit findings.
Part of this comes down to fostering a culture of compliance. An organization can achieve this through proper employee training. ETQ Reliance combines audit management with employee training tools to ensure everyone is up to speed on the latest regulatory changes. A central hub to store auditor profiles, training plans, and documentation streamlines the compliance program and reduces audit findings.
Monitoring, evaluation and continuous improvement
Regular audits should be welcomed by a company culture focused on continuous improvement. Proactive action and using tools such as ETQ Reliance allow an organization to always be audit ready. Regular internal audits should mimic a typical regulatory audit to help flag potential compliance issues. This drives home the idea that all areas of the organization could come under scrutiny from an auditor.
While a list of audit findings might feel like a setback, it’s actually an important part of continuous improvement. Knowing where issues are, it’s possible to address them and find ways to improve quality and compliance.
Achieving life sciences compliance
Elevate your life sciences compliance strategy and reduce audit findings with proactive, technology-enabled solutions. To learn more about how ETQ Reliance can help improve compliance at scale, look here.