ETQ DEVIATION MANAGEMENT
Perform each investigation consistently
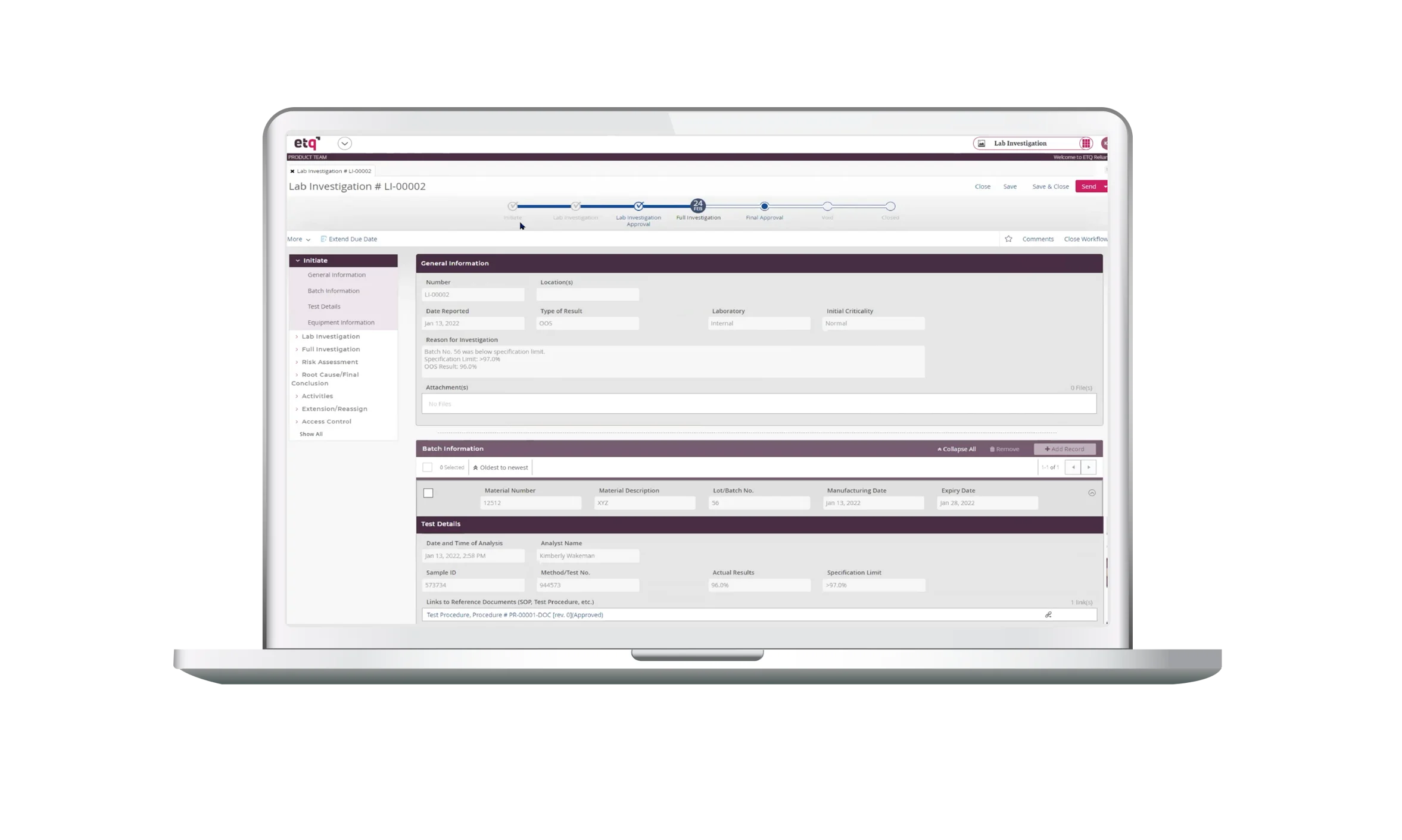
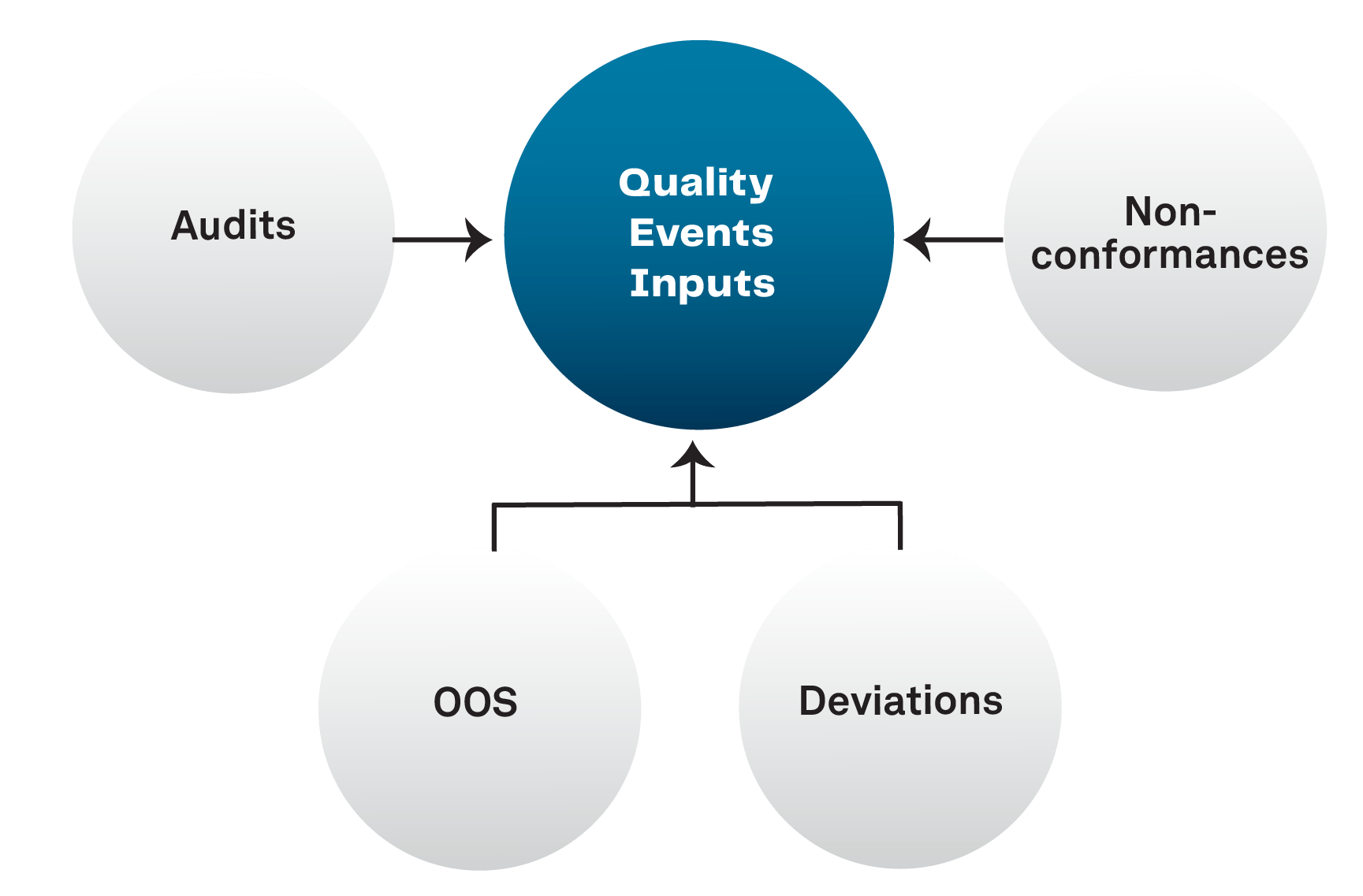
Quality Events is an application used to track & trend or manage the investigation process of a variety of unplanned events or deviations that impacts the quality, performance, safety, or reliability of a product or against cGMP.
- The app provides a standard process for investigating an event to ensure each investigation is performed consistently and in a timely manner.
- Organizations can discover trends that might not have otherwise been obvious. Quality Events is a central component to systematically detecting and correcting issues.