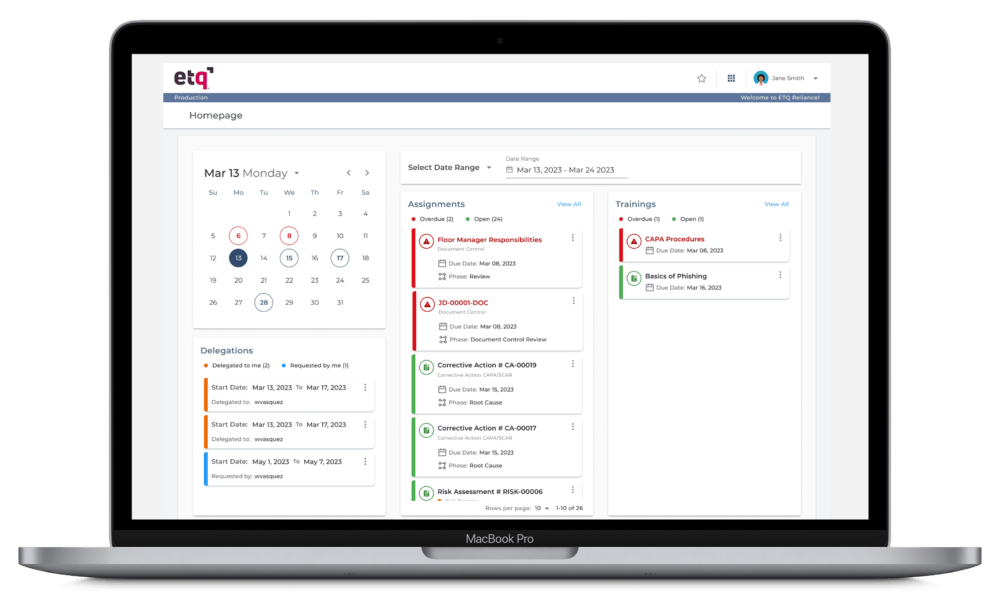
More than a quality management software
A quality management solution with all the resources quality experts need
- Quick and optimal – ETQ is your partner for a smooth and fast QMS implementation that matches your business’ needs and processes—today and in the future.
- On-demand training and a peer community – The only quality management system software that lets you learn at your own pace—and from other quality champions like you.
- Adaptable when your needs evolve – You can easily modify our QMS to make it just right for you. If you want support, our team of quality management systems professionals can offer a helping hand.